C-19 : Viral Transport Medium (VTM) for diagnosis
A Viral Transport Medium (VTM) following the WHO and CDC recommendations with or without swab
The success of the diagnosis of (2019-nCoV) during the C-19 outbreak depends largely on the quality of the specimen and the conditions under which the specimen is transported and stored before being processed in the laboratory.
Viral Transport Medium (VTM) allows the safe transfer of viruses, chlamydia and mycoplasma for further research, including conventional cell culture methods, diagnostic tests, and molecular biology techniques.
Commercially prepared viral transport media are available in a screw cap plastic tube containing buffered proteins (serum, albumin or gelatin) and antibiotics. Antibiotics are usually incorporated into the viral transport media to suppress the growth of contaminating bacteria and fungi, so separate samples from the same site should be collected if bacterial or fungal cultures are also requested.
Our Viral Transport Medium is based on Hanks Balanced Salt Solution (HBSS) with Calcium and Magnesium and contains heat-inactivated Fetal Bovine Serum, Gentamycin and Amphotericin B. The composition and the manufacturing of the Viral Transport Medium follow the WHO and the CDC recommendations.
The product is provided as a liquid in a sterile 13 ml flat bottom tube with or without a swab to provide a maximum range of possibilities to collect samples.
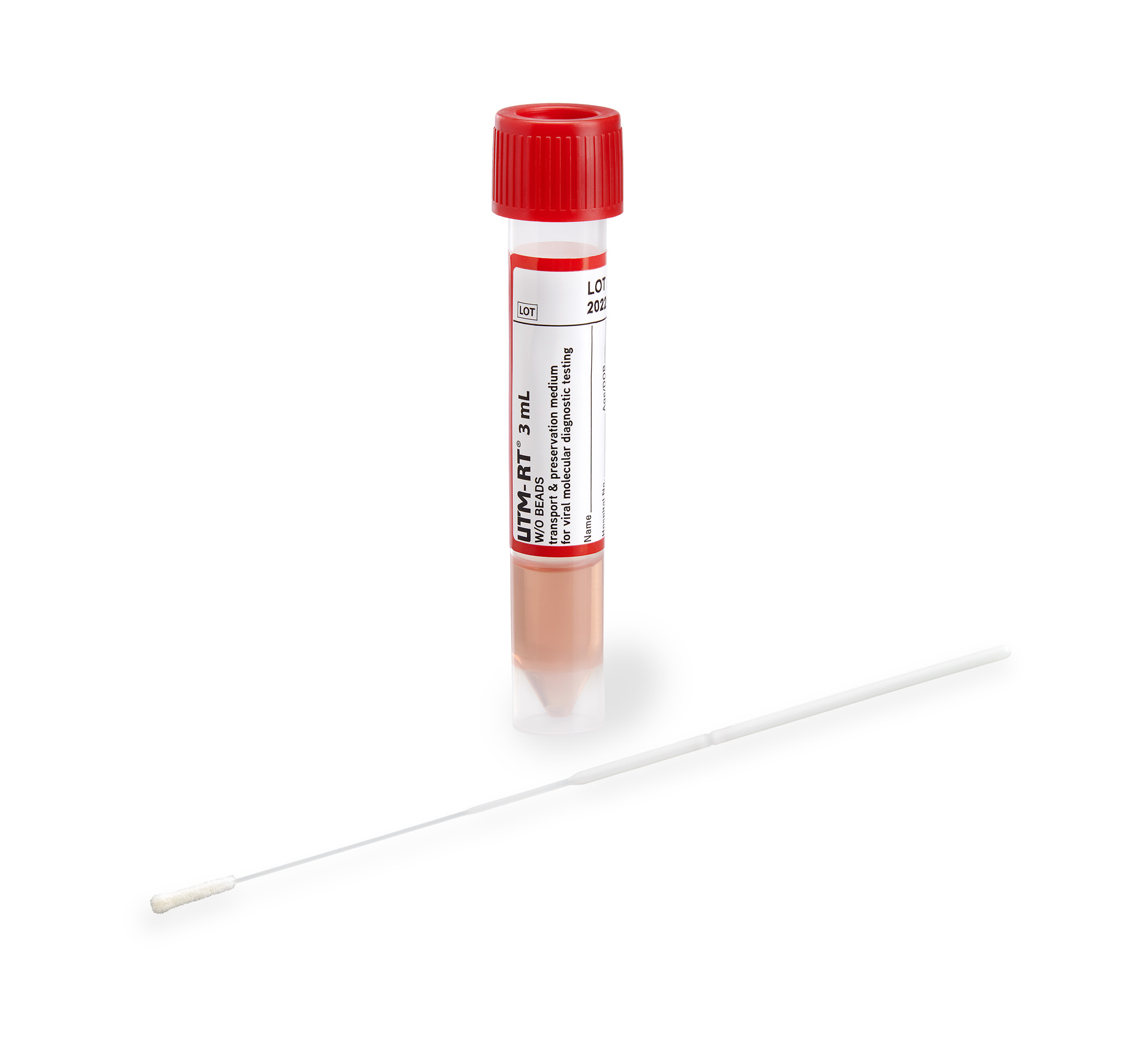
Charm Viral Transport Medium (VTM) is a non-propagating culture medium designed for the transport of clinical material. The intended use of VTM is for the transport of clinical samples for testing with biochemical, molecular, or antigen assays during the SARS-CoV-2 (COVID-19) public health emergency.
Charm VTM is manufactured following CDC SOP# DSR-052-05, Preparation of Viral Transport Medium per FDA Device List D411652 (JSG Code, Exempt).
Each polypropylene screw cap vial contains approximately 3 mL of sterile VTM.
Vials containing sample swabs and delivered refrigerated to laboratories can be used for testing for viruses, including COVID-19, by PCR, RNA/DNA extraction, antigen testing, or other validated diagnostic protocols.
Manufacturing and Availability
All VTM vials are manufactured at the Charm Sciences facility in the United States. Custom quantities and packaging available upon request.
Eliminate the need to order in bulk: Charm simplifies deliveries and quantities by arranging for automated shipments.
The VTM is available in 2 formats : Without and with swabs.
Description: Viral Transport Medium (VTM) Viral Transport Medium (VTM) with swabs
Product code: VTMK-49-3ML 49 vials of 3 ml + 49 swabs
Size: 49 vials of 3 ml 49 vials of 3 ml + 49 swabs
Medical devices for in vitro diagnosis. Read the operating instructions carefully.


