Rna vaccine
Recent improvements in mRNA vaccines act to increase protein translation, modulate innate and adaptive immunogenicity, and improve delivery.
MRNA vaccines have elicited potent immunity against infectious disease targets in animal models of influenza viruses, Zika viruses, rabies viruses, and others, especially in recent years, using lipid-encapsulated or naked forms of mRNA. optimized sequence.
Various approaches to cancer mRNA vaccines, including dendritic cell vaccines and various types of directly injectable mRNA, have been used in numerous cancer clinical trials, with some promising results showing antigen-specific T-cell responses and survival prolonged without disease in some cases.
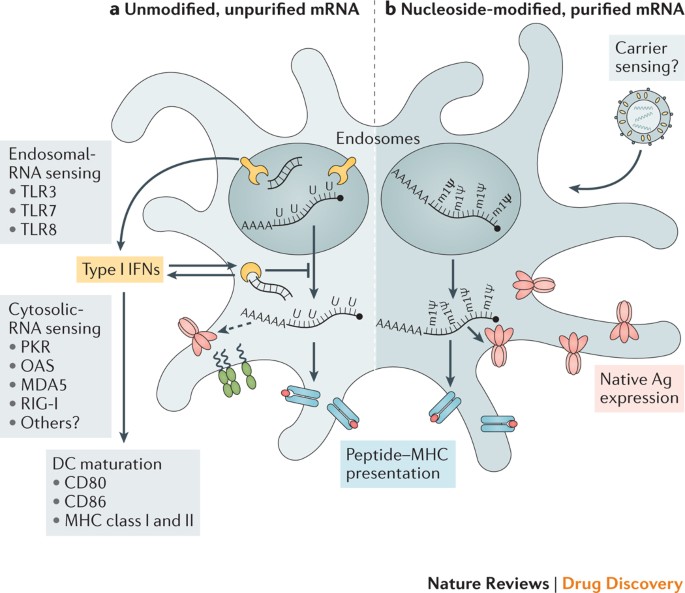
Therapeutic considerations and challenges include expanding Good Manufacturing Practice (GMP) production, establishing regulations, additional documentation of safety, and increasing efficacy.
Important future directions of research will be to compare and elucidate immune pathways activated by various mRNA vaccine platforms, improve current approaches based on these mechanisms, and initiate new clinical trials against additional disease targets.
Abstract
MRNA vaccines represent a promising alternative to conventional vaccine approaches due to their high potency, rapid development capabilities, and potential for low-cost manufacturing and safe administration. However, its application has been restricted until recently by instability and inefficient in vivo delivery of mRNA. Recent technological advances have largely overcome these problems, and multiple mRNA vaccine platforms against infectious diseases and various cancers have shown encouraging results in both animal and human models. This review provides a detailed description of mRNA vaccines and considers future directions and challenges in advancing this promising vaccine platform for widespread therapeutic use.
